Clopidogrel drug interactions: a review of the evidence and clinical implications
ABSTRACT
Introduction
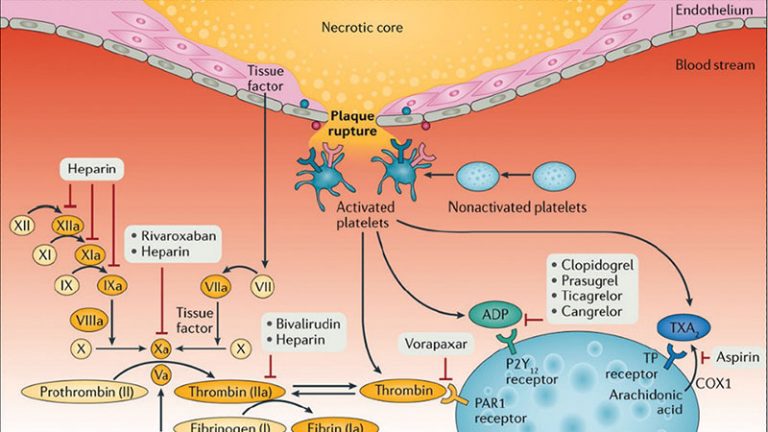
Patients with cardiovascular disease are commonly affected by a number of comorbidities leading to a high prevalence of polypharmacy. Polypharmacy increases the probability of drug–drug interactions (DDIs). Amongst these, DDIs involving clopidogrel, the most commonly utilized platelet P2Y12 inhibitor, is a topic of potential clinical concern.
Areas covered
This article reviews DDIs between clopidogrel and drugs which are widely used in clinical practice. In particular, drugs shown to interfere with the pharmacodynamic and pharmacokinetic effects of clopidogrel and the clinical implications of these findings are reviewed. These drugs include inhibitors of gastric acid secretion, statins, calcium channel blockers, antidiabetic agents, and antimicrobial agents. For the references, we searched PubMed, EMBASE, or the Cochrane Library.
Expert opinion
Clopidogrel–drug interactions are common. Most of these DDIs are limited to laboratory findings showing an impact on clopidogrel-induced antiplatelet effects. While variability in clopidogrel-induced antiplatelet effects is known to affect clinical outcomes, with high platelet reactivity being associated with thrombotic complications among patients undergoing coronary stenting, most studies assessing the clinical implications of clopidogrel–drug interactions have not shown to significantly affect outcomes. However, awareness of these DDIs remains important for optimizing the selection of concomitant therapies.
Article highlights
- Clopidogrel is the most commonly prescribed platelet P2Y12 inhibitor and because patients with cardiovascular disease are commonly affected by a number of comorbidities, polypharmacy is frequently encountered increasing the risk of drug–drug interactions (DDIs).
- Clopidogrel drug-drug interactions occur with agents sharing the same metabolic pathway which can thus interfere with clopidogrel’s pharmacokinetic and pharmacodynamic profiles, and a reduction in the clopidogrel-induced antiplatelet effects as a result of DDIs may increase the risk of thrombotic complications.
- Clopidogrel can also interfere with the metabolism of other drugs, thereby lowering their efficacy or causing adverse effect.
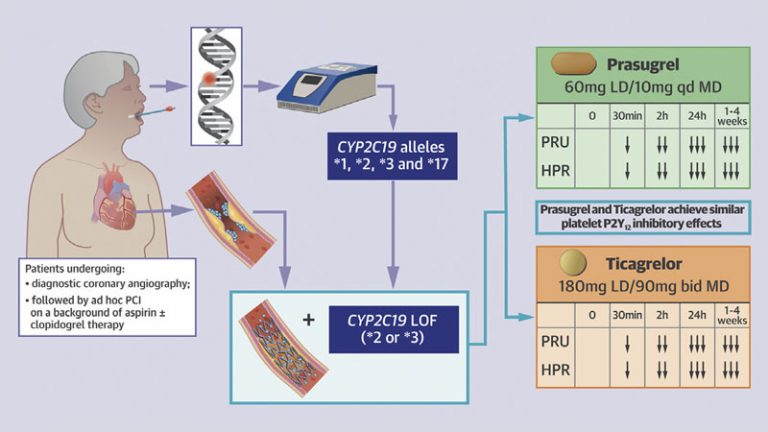
- Proton pump inhibitors (PPIs), particularly those interfering with CYP2C19 activity, the key enzyme involved in clopidogrel metabolism, have shown to affect the pharmacokinetic and pharmacodynamic profile of clopidogrel leading to a box warning from drug regulating agencies on the clopidogrel product label.
- Despite the established DDI between clopidogrel and PPIs, the clinical implications associated with concomitant use of these drugs have been controversial with practice guidelines recommending the use of PPIs in patients at high risk for bleeding.
- A number of other DDIs have been described including certain statins (e.g. atorvastatin and simvastatin), calcium channel blockers (e.g. amlodipine), glucose lowering drugs (e.g. sulfonylureas) and antivirals (e.g. ritonavir) which share some of the same steps of the metabolic pathway as clopidogrel.
This box summarizes key points contained in the article.
Declaration of interest
F Franchi has received payment as an individual for consulting fee or honorarium from AstraZeneca Bayer and Sanofi. D. J. Angiolillo has received payment as an individual for: reports receiving payments as an individual for: a) Consulting fee or honorarium from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; b) Participation in review activities from CeloNova and St. Jude Medical. Institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli-Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions and the Scott R. MacKenzie Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.