Pharmacodynamic and Pharmacokinetic of Switching From Cangrelor to Prasugrel in ACS Patients Undergoing PCI
Overview
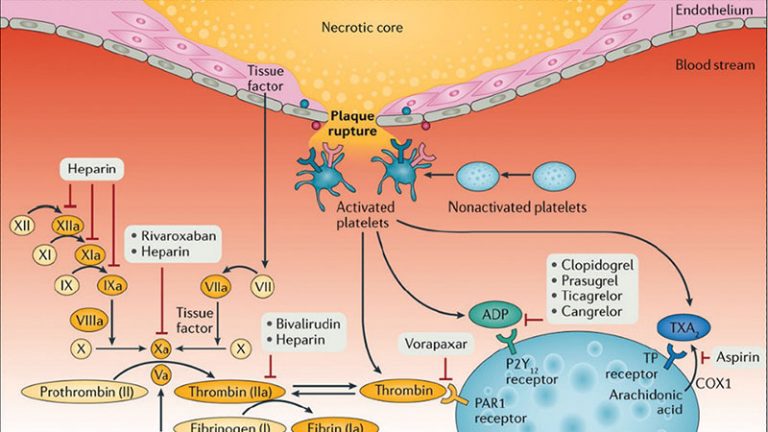
Cangrelor is an intravenous P2Y12 inhibitor utilized as a bridge to achieve adequate platelet inhibition until oral P2Y12 inhibitors achieve their full antiplatelet effects in patients undergoing coronary stenting. Although in this setting the potent oral P2Y12 inhibitor prasugrel is commonly utilized, there is very limited data on the optimal approach for switching between these therapies. The overarching aim of this investigation is to rule out a drug drug interaction (DDI) when cangrelor and prasugrel are concomitantly administered in patients undergoing coronary stenting.
Full Title of Study: “Pharmacodynamic and Pharmacokinetic Profiles on Switching From Cangrelor to Prasugrel in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention: The Switching Antiplatelet -6 (SWAP-6) Study”
Study Type
- Study Type: Interventional
- Study Design
- Allocation: Randomized
- Intervention Model: Parallel Assignment
- Primary Purpose: Treatment
- Masking: None (Open Label)
- Study Primary Completion Date: September 1, 2022
Detailed Description
Cangrelor is an intravenous P2Y12 inhibitor utilized as a bridge to achieve adequate platelet inhibition until oral P2Y12 inhibitors achieve their full antiplatelet effects in patients undergoing coronary stenting. Although in this setting the potent oral P2Y12 inhibitor prasugrel is commonly utilized, there is very limited data on the optimal approach for switching between these therapies. In particular, ruling out a drug-drug interaction (DDI) is critical to this extent as the presence of a DDI would translate into reduced or abolished antiplatelet effects exposing these acute patients to an increased thrombotic risk. There is an unmet need to better elucidate pharmacodynamic profiles associated with the transition from cangrelor to prasugrel therapy. Of note, prasugrel has recently gone off patent and the availability of a generic formulation will favorably impact its use. Pharmacodynamic studies provide some support on the safety of administering prasugrel at the start of cangrelor infusion. However, the available data does not allow to rule out a DDI given that there was no comparator arm in which prasugrel was either given alone or at the end of cangrelor infusion. The methodological approach for this assessment should rely on comprehensive pharmacodynamics investigations aimed to assess levels of P2Y12 receptor inhibition, pharmacokinetic investigations to assess systemic levels of the drug/drug metabolite, and mechanistic investigations by assessment of levels of P2Y12 receptor gene expression. The overarching aim of this investigation is to rule out a DDI when cangrelor and prasugrel are concomitantly administered in patients undergoing coronary stenting.
Interventions
- Drug: Cangrelor
- Cangrelor will be used at the FDA recommended dose using a 30 μg/kg bolus followed by 4 μg/kg/min infusion. The total infusion will last 2 hours.
- Drug: Prasugrel
- Prasugrel will be used in line with FDA recommendations using a 60mg LD followed by a 10mg daily maintenance dose started 24 hours after LD administration
Arms, Groups and Cohorts
- Active Comparator: Prasugrel
- Prasugrel only administered at the start of PCI
- Experimental: Prasugrel + Cangrelor
- Cangrelor plus prasugrel concomitantly administered at the start of PCI
- Active Comparator: Cangrelor followed by Prasugrel
- Cangrelor administered at the start of PCI plus prasugrel administered at the end of the cangrelor infusion
Clinical Trial Outcome Measures
Primary Measures
- Platelet reactivity measured by VerifyNow
- Time Frame: 4 hours
- The primary end point of the study will be the non-inferiority in P2Y12 reaction units (PRU) of cangrelor plus prasugrel concomitantly administered at the start of PCI versus prasugrel only administered at the start of PCI.
Participating in This Clinical Trial
Inclusion Criteria
- Patients with NSTE-ACS (UA or NSTEMI) undergoing PCI. NSTE-ACS will be defined as the presence of cardiac ischemic symptoms with ischemic changes (but not ST-segment elevation) on electrocardiogram with or without a positive troponin. However, normal electrocardiograms will be acceptable if the investigator will consider an ACS presentation likely. – Age between 18 and 75 years old Exclusion Criteria:
- Inability to provide written informed consent – Age >75 years – Weight <60 Kg – ST-segment elevation myocardial infarction – On treatment with a P2Y12 receptor antagonist (ticlopidine, clopidogrel, prasugrel, ticagrelor) in past 7 days – Known allergies to prasugrel or cangrelor – Considered at high risk for bleeding – History of ischemic or hemorrhagic stroke or transient ischemic attack – On treatment with oral anticoagulant (Vitamin K antagonists, dabigatran, rivaroxaban, apixaban, edoxoban) – Planned treatment with glycoprotein IIb/IIIa inhibitors (only bailout use allowed) – Fibrinolytics within 24 hours – Known platelet count <80×106/mL – Known hemoglobin <10 g/dL – Active bleeding – Known end stage renal disease on hemodialysis – Known severe hepatic dysfunction – Intubated patients (prior to randomization) – Pregnant females [women of childbearing age must use reliable birth control (i.e. oral contraceptives) while participating in the study]
Gender Eligibility: All
Minimum Age: 18 Years
Maximum Age: 75 Years
Are Healthy Volunteers Accepted: No
Investigator Details
- Lead Sponsor
- University of Florida
- Collaborator
- Scott R MacKenzie Foundation
- Provider of Information About this Clinical Study
- Sponsor
- Overall Official(s)
- Francesco Franchi, MD, Principal Investigator, University of Florida
- Overall Contact(s)
- Francesco Franchi, MD, 904-244-2060, francesco.franchi@jax.ufl.edu