Switching Between Intravenous and Oral P2Y12 Inhibition: Easier Said Than Done
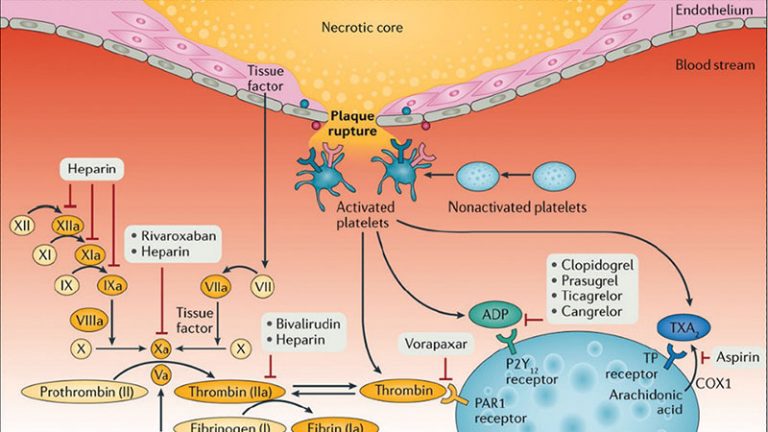
Adequate suppression of platelet reactivity is necessary to mitigate early thrombotic events, including stent thrombosis, among patient undergoing percutaneous coronary intervention (PCI). Clinical trials conducted more than 20 years ago established the superiority of adding an oral P2Y12 inhibitor to aspirin, compared with alternative antithrombotic strategies, in preventing ischemic events among patients with stable or acute coronary syndromes (ACS) undergoing PCI.1,2 Nonetheless, the inhibitory effects of oral antiplatelet agents may be attenuated or delayed in the setting of critical illness, ST-segment elevation myocardial infarction (STEMI) presentation, or genetic polymorphisms that influence pharmacodynamic (PD) effect.3,4 Although glycoprotein IIb/IIIa inhibitors may overcome these limitations, bleeding risk associated with these agents precludes routine use during PCI. In contrast, cangrelor is a direct-acting, potent parenteral P2Y12 antagonist characterized by a very short half-life with a quick onset and offset of action.5 Compared with clopidogrel, cangrelor significantly reduced thrombotic events among patients undergoing PCI who were naive to P2Y12 inhibition.6
The PD effects of the orally administered thienopyridines clopidogrel and prasugrel are blunted when given concurrently with cangrelor.7,8 The underlying mechanism for this adverse drug-drug interaction (DDI) centers on high P2Y12 receptor occupancy achieved with cangrelor that impedes the active metabolites of either drug from receptor binding, thereby culminating in rapid elimination or extravascular distribution, respectively. By contrast, ticagrelor binds to a distinct site on the P2Y12 receptor and its active metabolite is characterized by a relatively long half-life, rendering a DDI less likely.9,10 This issue is clinically relevant, as ticagrelor-induced platelet inhibition is delayed in the setting of ACS,3 highlighting the need for adjunctive platelet inhibition. Moreover, although cangrelor is not uncommonly given to patients pretreated with ticagrelor,11, 12, 13 the PD effects of such a strategy are unclear.
To address these evidence gaps, Franchi et al14 report findings from the SWAP-5 (Switching Antiplatelet Therapy-5) study in this issue of JACC: Cardiovascular Interventions. The stated objective of this single-center pharmacokinetic (PK)/PD study was to confirm or refute the presence of a DDI when cangrelor is administered in the setting of ticagrelor pretreatment. The randomized, double-blind, placebo-controlled crossover study included patients with stable Coronary artery disease (n = 20, mean age 64 years, 55% female) not receiving oral P2Y12 inhibitors. Patients were randomly allocated to receive either cangrelor (bolus plus 2-hour infusion) or placebo 1 hour after a 180-mg loading dose of ticagrelor. The primary endpoint was residual platelet reactivity measured 2 hours after completion of the cangrelor/placebo infusion and was quantified using the VerifyNow system. High platelet reactivity (HPR) was defined as >208 P2Y12 reaction units. The investigators hypothesized that the effect of ticagrelor alone on the primary endpoint would be noninferior to that of ticagrelor plus cangrelor. PD results showed that platelet inhibition was enhanced with cangrelor at 30 minutes and 1 hour after initiation compared with ticagrelor alone, although differences were smaller in magnitude thereafter. With respect to the primary endpoint, P2Y12 reaction units measured 2 hours after discontinuation of cangrelor or placebo infusion were low and similar between groups (16.9 vs 12.6; 95% CI: −28.6 to 37.3), with a difference below the noninferiority margin of 45. The results were consistent using alternative PD measurements. Importantly, PK results were concordant with the PD findings, showing no differences in ticagrelor active metabolite at different time points between groups. These results, coupled with earlier data, suggest that ticagrelor-induced platelet inhibition is not modified by cangrelor, thereby ruling out a DDI between these agents.15,16 This validity of this result is strengthened by the robust study design, multiple assays used to evaluate platelet reactivity, and PK measurements.
The SWAP-5 results also provide novel and mechanistic insights regarding the potential role of adjunctive cangrelor on a background of strong P2Y12 inhibition. Indeed, the clinical efficacy of cangrelor has been established only against a clopidogrel comparator among patients naive to P2Y12 inhibition.6 Moreover, the role of cangrelor should be examined in the broader context of alternative therapeutic strategies that enhance platelet inhibition,17,18 or lower thrombotic risk19 at the time of PCI. To examine these issues, Gargiulo et al20 compared the PD effects of prasugrel, glycoprotein IIb/IIIa inhibition with tirofiban, and cangrelor among patients with STEMI undergoing PCI. The investigators found that platelet inhibition was highest with tirofiban, intermediate with cangrelor, and lowest with prasugrel. In a similar cohort treated with crushed ticagrelor, Franchi et al15 found that concomitant cangrelor reduced HPR rates by an absolute 60% compared with ticagrelor alone after 30 minutes. In contrast, cangrelor yielded a more modest 10% reduction in HPR at a similar time point among stable SWAP-5 participants pretreated with ticagrelor. Although somewhat discordant, these results suggest that the PD effects of cangrelor, and by extension putative clinical benefit, will vary in relation to baseline P2Y12 receptor status (naive vs non-naive), clinical presentation, and timing of administration. As pointed out by the investigators, this hypothesis remains speculative in the absence of an adequately powered randomized trial evaluating the effect of cangrelor in patients with ACS receiving contemporary pharmacotherapy.
Absent clear evidence supporting the use of cangrelor in the setting of ACS and strong P2Y12 inhibition, it is not surprising that practice-based use is highly variable and often discordant with regulatory labeling.11,12 For example, Rymer et al11 found that hospital-level cangrelor use among patients with ACS varied between 6% and 100%.11 Univariable factors associated with greater cangrelor use included STEMI presentation, active smoking, angiographic thrombus, and non-Hispanic ethnicity. The investigators also observed that 16% of cangrelor-treated patients had at least a 1-hour gap in the initiation of an oral P2Y12 inhibitor. Even more concerning, this delay was most pronounced among patients transitioning to clopidogrel vs prasugrel or ticagrelor (56.6% vs 34.5% vs 10.8%; P < 0.001). Reasons for these treatment delays are unclear but might reflect implementation gaps during transitions of care from the catheterization laboratory to inpatient ward. Irrespective of underlying reason, these results suggest a significant proportion of patients with ACS receiving cangrelor in contemporary practice remain susceptible to recurrent thrombosis because of poor adherence to evidence-based recommendations and labeling instructions. Hence, although Franchi et al14 have shown us that switching from cangrelor to ticagrelor (or vice versa) is tenable and appears straightforward in the controlled setting of a randomized trial, clinical implementation in usual-care settings must be improved upon to avoid interruptions in platelet inhibition and to ensure optimal patient outcomes.
Funding Support and Author Disclosures
The author has reported that he has no relationships relevant to the contents of this paper to disclose.
References
- S.R. Mehta, S. Yusuf, R.J. Peters, et al.
Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study
Lancet, 358 (2001), pp. 527-533
View PDF View article View in Scopus Google Scholar - M.B. Leon, D.S. Baim, J.J. Popma, et al., for the Stent Anticoagulation Restenosis Study Investigators
A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting
N Engl J Med, 339 (1998), pp. 1665-1671
View in Scopus Google Scholar - D. Alexopoulos, I. Xanthopoulou, V. Gkizas, et al.
Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction
Circ Cardiovasc Interv, 5 (2012), pp. 797-804
View in Scopus Google Scholar - D.J. Angiolillo, A. Fernandez-Ortiz, E. Bernardo, et al.
Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives
J Am Coll Cardiol, 49 (2007), pp. 1505-1516
View PDF View article View in Scopus Google Scholar - W.S. Akers, J.J. Oh, J.H. Oestreich, S. Ferraris, M. Wethington, S.R. Steinhubl
Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist
J Clin Pharmacol, 50 (2010), pp. 27-35
View at publisher CrossRef View in Scopus Google Scholar - D.L. Bhatt, G.W. Stone, K.W. Mahaffey, et al.
Effect of platelet inhibition with cangrelor during PCI on ischemic events
N Engl J Med, 368 (2013), pp. 1303-1313
View at publisher CrossRef View in Scopus Google Scholar - S.R. Steinhubl, J.J. Oh, J.H. Oestreich, S. Ferraris, R. Charnigo, W.S. Akers
Transitioning patients from cangrelor to clopidogrel: pharmacodynamic evidence of a competitive effect
Thromb Res, 121 (2008), pp. 527-534
View PDFView articleView in ScopusGoogle Scholar - D.J. Schneider, N. Seecheran, S.S. Raza, F.K. Keating, P. Gogo
Pharmacodynamic effects during the transition between cangrelor and prasugrel
Coron Artery Dis, 26 (2015), pp. 42-48
View in ScopusGoogle Scholar - S. Husted, J.J. van Giezen
Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist
Cardiovasc Ther, 27 (2009), pp. 259-274
View at publisher CrossRefView in ScopusGoogle Scholar - D.J. Angiolillo, F. Rollini, R.F. Storey, et al.
International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies
Circulation, 136 (2017), pp. 1955-1975
View in ScopusGoogle Scholar - J.A. Rymer, D.L. Bhatt, D.J. Angiolillo, et al.
Cangrelor use patterns and transition to oral P2Y12 inhibitors among patients with myocardial infarction: initial results from the CAMEO registry
J Am Heart Assoc, 11 (2022), Article e024513
View in ScopusGoogle Scholar - P. Grimfjard, B. Lagerqvist, D. Erlinge, C. Varenhorst, S. James
Clinical use of cangrelor: nationwide experience from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR)
Eur Heart J Cardiovasc Pharmacother, 5 (2019), pp. 151-157
View at publisher CrossRefView in ScopusGoogle Scholar - M. Vaduganathan, A. Qamar, A. Singh, et al.
Cangrelor use since FDA approval: a single-center, real-world experience at a tertiary care hospital
J Am Coll Cardiol, 69 (2017), pp. 463-464
View PDFView articleView in ScopusGoogle Scholar - F. Franchi, L. Ortega-Paz, F. Rollini, et al.
Cangrelor in patients with coronary artery disease pretreated with ticagrelor: the switching antiplatelet (SWAP)-5 study
J Am Coll Cardiol Intv, 16 (2023), pp. 36-46
View PDFView articleView in ScopusGoogle Scholar - F. Franchi, F. Rollini, A. Rivas, et al.
Platelet inhibition with cangrelor and crushed ticagrelor in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention
Circulation, 139 (2019), pp. 1661-1670
View at publisher CrossRefView in ScopusGoogle Scholar - D.J. Schneider, Z. Agarwal, N. Seecheran, F.K. Keating, P. Gogo
Pharmacodynamic effects during the transition between cangrelor and ticagrelor
J Am Coll Cardiol Intv, 7 (2014), pp. 435-442
View PDFView articleView in ScopusGoogle Scholar - G. Parodi, I. Xanthopoulou, B. Bellandi, et al.
Ticagrelor crushed tablets administration in STEMI patients: the MOJITO study
J Am Coll Cardiol, 65 (2015), pp. 511-512
View PDFView articleView in ScopusGoogle Scholar - M. Valgimigli, M. Tebaldi, G. Campo, et al.
Prasugrel versus tirofiban bolus with or without short post-bolus infusion with or without concomitant prasugrel administration in patients with myocardial infarction undergoing coronary stenting: the FABOLUS PRO (Facilitation Through Aggrastat by Dropping or Shortening Infusion Line in Patients With ST-Segment Elevation Myocardial Infarction Compared to or on Top of Prasugrel Given at Loading Dose) trial
J Am Coll Cardiol Intv, 5 (2012), pp. 268-277
View PDFView articleView in ScopusGoogle Scholar - Y. Li, Z. Liang, L. Qin, et al.
Bivalirudin plus a high-dose infusion versus heparin monotherapy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: a randomised trial
Lancet, 400 (10366) (2022), pp. 1847-1857
View PDFView articleView in ScopusGoogle Scholar - G. Gargiulo, G. Esposito, M. Avvedimento, et al.
Cangrelor, tirofiban, and chewed or standard prasugrel regimens in patients with ST-segment-elevation myocardial infarction: primary results of the FABOLUS-FASTER trial
Circulation, 142 (2020), pp. 441-454
View at publisher CrossRefView in ScopusGoogle Scholar